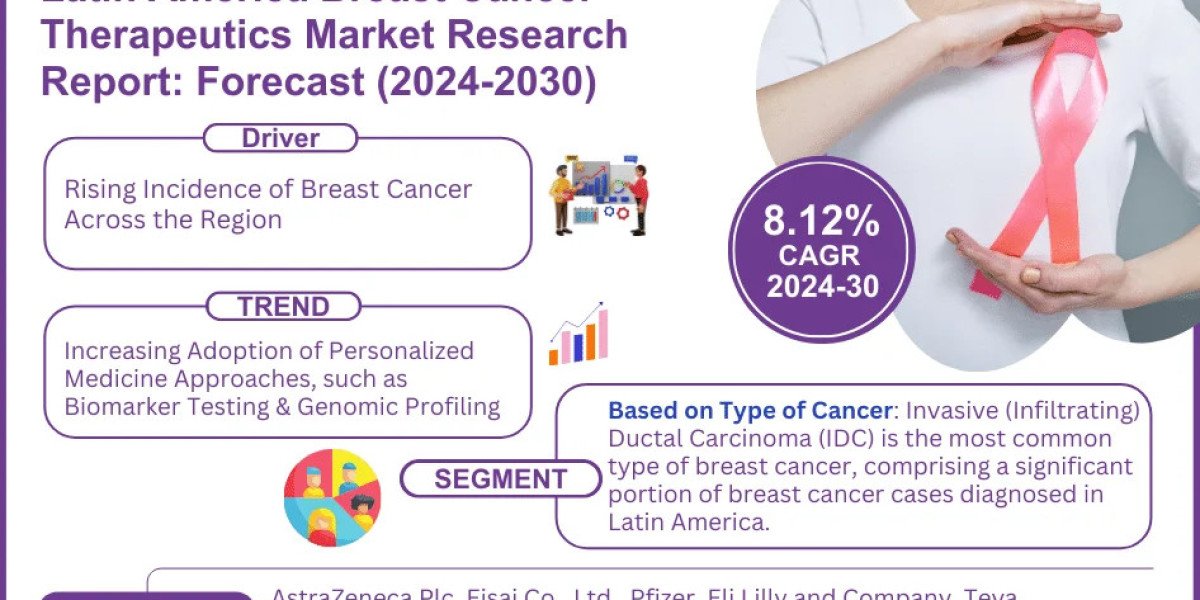
The pharmaceutical industry is dynamic and ever-evolving, particularly when it comes to regulatory compliance . As we enter 2024, the UK has introduced several significant updates to its pharmaceutical regulations that industry stakeholders need to be aware of. Navigating these regulatory waters can be challenging, but understanding the key updates can help ensure compliance and maintain market access. Here's a comprehensive overview of the most critical changes and their implications for pharmaceutical companies.
- New MHRA Guidelines on Clinical Trials
The Medicines and Healthcare products Regulatory Agency (MHRA) has introduced updated guidelines for clinical trials to enhance patient safety and streamline processes. These guidelines emphasize the importance of robust data collection and analysis to ensure the reliability of clinical trial outcomes. Key changes include:
- Simplified Application Process: The MHRA has streamlined the clinical trial application process, reducing administrative burdens and expediting approval times.
- Enhanced Patient Safety Protocols: New requirements for monitoring and reporting adverse events have been established, ensuring quicker responses to potential safety issues.
- Greater Transparency: Sponsors must now provide more comprehensive information about ongoing trials, promoting transparency and public trust.
- Brexit-Driven Regulatory Divergence
Since Brexit, the UK has been gradually diverging from EU regulations. In 2024, several new regulatory affairs frameworks specific to the UK will come into effect. These include:
- UKCA Marking: Products marketed in the UK will need the UK Conformity Assessed (UKCA) marking instead of the CE marking. This change requires manufacturers to ensure their products meet UK-specific standards.
- Innovative Licensing and Access Pathway (ILAP): This new pathway aims to accelerate the development and delivery of innovative medicines to patients by providing enhanced regulatory support throughout the product lifecycle.
- Focus on Digital Health Technologies
The integration of digital health technologies in pharmaceutical care is rapidly growing. The MHRA has recognized this trend and updated its guidelines to ensure these technologies are safe and effective. Key updates include:
- Regulation of Health Apps: Digital health applications must now undergo rigorous assessment to ensure they meet the necessary standards for data security, patient safety, and clinical efficacy.
- AI and Machine Learning: New guidelines have been introduced for AI and machine learning-based medical devices , focusing on transparency, performance monitoring, and risk management.
- Sustainability in Pharmaceuticals
In line with global sustainability goals, the UK is implementing stricter environmental regulations for pharmaceutical companies. These changes are aimed at reducing the environmental impact of pharmaceutical manufacturing and disposal. Key initiatives include:
- Green Manufacturing Practices: Pharmaceutical companies are encouraged to adopt green manufacturing practices, including reducing waste, conserving energy, and using environmentally friendly materials.
- Safe Disposal of Medicines: New guidelines for the safe disposal of medicines have been introduced to prevent environmental contamination and promote public health.
- Enhanced Pharmacovigilance Requirements
Pharmacovigilance—the monitoring of the safety of medicines after they have been released on the market—remains a top priority for the MHRA. In 2024, enhanced requirements for pharmacovigilance services include:
- Real-Time Data Reporting: Companies must implement real-time data reporting systems to quickly identify and respond to adverse drug reactions.
- Risk Management Plans: Updated guidelines on risk management plans (RMPs) require more detailed strategies for mitigating risks associated with pharmaceutical products.
Conclusion
Staying abreast of regulatory changes is crucial for pharmaceutical companies operating in the UK. The updates for 2024 reflect a broader trend towards increased transparency, patient safety, and environmental responsibility. By understanding and adhering to these new regulations, pharmaceutical companies can ensure compliance, maintain market access, and continue to innovate in a rapidly changing industry.
Navigating these regulatory waters may seem daunting, but with the right knowledge and preparation, companies can successfully adapt to these changes and thrive in the UK market. For ongoing updates and detailed guidance, always refer to official MHRA publications and consult with regulatory experts.
For more information, visit regulatory services in USA , medical device services , and medical writing .