The North American clinical trials market is a significant segment of the global healthcare and pharmaceutical industries, facilitating the development of new and innovative therapies. Valued at USD 35.31 billion in 2024, the market is projected to grow at a compound annual growth rate (CAGR) of 6.0% during the forecast period from 2025 to 2034, reaching a value of USD 60.16 billion by 2034. The growth of the market is driven by the increasing number of clinical trials, the rising demand for new therapies, and advancements in technology that streamline trial processes. North America, with its advanced healthcare infrastructure, regulatory frameworks, and a high number of pharmaceutical companies, remains a leading region for clinical trials.
Market Overview
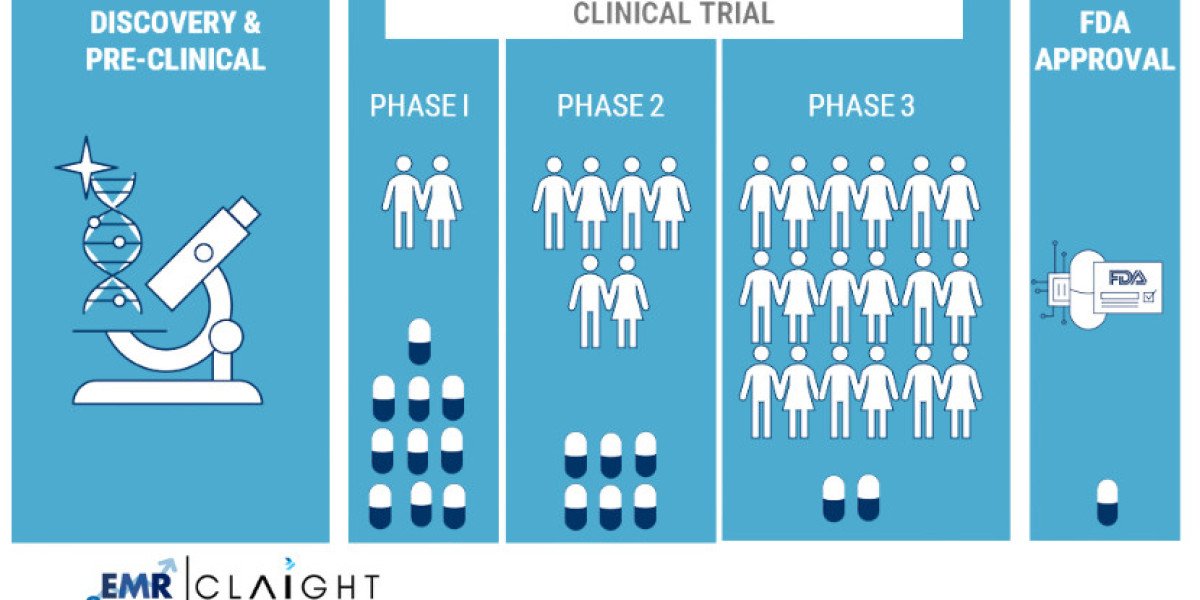
Clinical trials are essential for testing the safety and efficacy of new drugs, biologics, medical devices, and therapies. These trials follow a structured process of different phases, including Phase I to Phase IV, to evaluate a product’s effectiveness and side effects. North America, particularly the United States and Canada, leads the North America Clinical Trials Market due to a favorable regulatory environment, the strong presence of biopharmaceutical companies, and world-class research institutions. Furthermore, increasing investment in healthcare research and development, a growing demand for personalized medicine, and expanding patient populations contribute significantly to market growth in the region.
Market Size and Share
The North American clinical trials market was valued at USD 35.31 billion in 2024, with strong growth projected over the next decade. The market is expected to grow at a CAGR of 6.0%, reaching a value of USD 60.16 billion by 2034. The market’s expansion is driven by increasing investments in healthcare R&D, advancements in clinical trial technologies, and a large pool of patients willing to participate in clinical studies. In addition, the rise in chronic diseases, and cancer cases, and the growing focus on personalized and precision medicine contribute to the demand for clinical trials.
Market Trends
Growth of Decentralized Clinical Trials
Decentralized clinical trials (DCTs) are becoming increasingly popular due to their ability to improve patient access and retention. With the use of digital health technologies such as telemedicine, wearable devices, and mobile health apps, DCTs allow for remote patient monitoring, reducing the need for in-person visits. This trend is expected to accelerate as more pharmaceutical companies adopt decentralized approaches to conduct clinical trials, improving efficiency and patient convenience.
Rise in Oncology Clinical Trials
Oncology remains one of the most active therapeutic areas in clinical trials. With cancer cases on the rise globally, the need for innovative therapies is greater than ever. North America, with its well-established healthcare system and extensive research infrastructure, continues to lead the way in oncology clinical trials. Immunotherapy, gene therapies, and personalized medicine are some of the emerging trends that are driving the growth of clinical trials in oncology.
Technological Advancements in Data Management
Technological advancements are revolutionizing the clinical trials market, especially in the area of data management. Cloud-based platforms, AI-powered analytics, and electronic data capture systems are improving the efficiency of clinical trial processes, enhancing data accuracy, and ensuring compliance with regulatory standards. The use of technology in clinical trials is helping streamline recruitment, monitoring, and reporting, making it a key trend in the market’s growth.
Increased Focus on Rare Diseases and Personalized Medicine
The focus on rare diseases and personalized medicine is driving innovation in clinical trials. As the demand for treatments tailored to individual genetic profiles grows, pharmaceutical companies are conducting more clinical trials aimed at developing therapies for rare and genetic diseases. This shift towards personalized medicine is creating new opportunities for clinical trials in North America, particularly with the rise in gene therapies and novel biologics.
Get a Free Sample Report with Table of Contents
Market Analysis
Service Type: Laboratory and Testing Services Lead
The North American clinical trials market is divided into various service types, with laboratory services, bioanalytical testing services, and decentralized clinical trial services taking the lead. The need for accurate and efficient testing in clinical trials is propelling the demand for laboratory services. Additionally, bioanalytical testing services play a vital role in ensuring the quality and safety of drugs under investigation. As the market evolves, decentralized clinical trials (DCTs) are expected to gain more traction due to their flexibility and ability to reach patients remotely.
Phase: Phase III and IV Trials Dominate
Phase III and Phase IV trials are the most critical stages in clinical trials, contributing significantly to the market share. Phase III trials are pivotal in determining the efficacy and safety of a drug in large patient populations, while Phase IV trials focus on post-market surveillance to monitor long-term safety and effectiveness. The dominance of these phases is expected to continue as more drugs advance through the clinical trial pipeline in the coming years.
Therapeutic Area: Oncology and Neurology Drive Growth
Oncology and neurology are two of the most significant therapeutic areas driving clinical trial activities in North America. As cancer rates continue to rise, the need for more effective cancer treatments, including immunotherapies and biologics, is propelling clinical trials in oncology. Neurological disorders such as Alzheimer’s, Parkinson’s, and multiple sclerosis also contribute to the market growth, with significant investments directed toward novel treatments and therapies for these conditions.
Application: Small Molecules and Monoclonal Antibodies
Small molecules and monoclonal antibodies remain dominant in clinical trial applications in North America. Small molecules, widely used in drug development, continue to be essential in treating a variety of diseases. Similarly, monoclonal antibodies, which have shown significant promise in cancer treatment and immunotherapy, are gaining increasing attention. With advancements in biotechnology, both small molecules and monoclonal antibodies are expected to remain central in clinical trials over the next decade.
Scope of the Report
This report provides an in-depth analysis of the North American clinical trials market, including historical trends, future market forecasts, and an exploration of key industry drivers and challenges. The report also includes detailed segmentation by service type, clinical trial phase, therapeutic area, application, and region. Furthermore, it highlights emerging trends such as decentralized clinical trials, technological advancements in data management, and the shift toward personalized medicine.
Regional Insights
United States
The United States remains the dominant player in the North American clinical trials market due to its robust healthcare infrastructure, a large number of pharmaceutical and biotechnology companies, and a significant patient population. The U.S. also benefits from a favorable regulatory environment, led by the Food and Drug Administration (FDA), which accelerates the approval process for innovative therapies. The country’s well-established clinical trial ecosystem continues to attract investment, making it a primary location for clinical research and development.
Canada
Canada has also seen significant growth in the clinical trials market, supported by its highly skilled workforce, advanced healthcare system, and research expertise. Canadian institutions are involved in numerous clinical trials, especially in oncology, neurology, and metabolic disorders. The regulatory framework in Canada is aligned with international standards, ensuring smooth clinical trial operations. Additionally, Canada’s diverse patient population offers a wide range of clinical trial participants, enhancing the effectiveness of trials in the region.
Market Growth
The North American clinical trials market is poised for significant growth, driven by several key factors. The rise in chronic diseases such as cancer, diabetes, and neurological disorders increases the demand for new treatments, further spurring the need for clinical trials. Technological innovations such as AI, machine learning, and decentralized clinical trials are also enhancing the efficiency and accessibility of clinical research. Moreover, growing investments in personalized medicine and gene therapies are creating new opportunities for clinical trials, driving market expansion.
Recent Developments & Challenges
Recent developments in the clinical trials market include the widespread adoption of decentralized clinical trials, enabling broader patient participation and reducing trial costs. Innovations in data analytics and AI are enhancing trial design, patient recruitment, and monitoring, making trials more efficient. However, the industry faces challenges such as regulatory complexities, patient recruitment hurdles, and the high cost of conducting trials. Addressing these challenges will be key to maintaining growth in the clinical trials market.
Key Players
IQVIA Inc.
IQVIA Inc. is a leading global provider of advanced analytics, technology solutions, and contract research services. The company plays a significant role in the North American clinical trials market, offering comprehensive clinical trial services ranging from patient recruitment to data management and analytics. IQVIA’s innovative solutions and expertise in clinical research have positioned it as a key player in accelerating the clinical development process.
Laboratory Corporation of America Holdings
Laboratory Corporation of America Holdings (LabCorp) is a prominent provider of diagnostic testing and clinical trial services. The company offers laboratory services, clinical testing, and data management solutions to support clinical trials. LabCorp’s extensive network and expertise in clinical research make it a valuable partner for pharmaceutical companies conducting trials across North America.
Syneos Health
Syneos Health is a leading biopharmaceutical solutions organization offering a range of clinical trial services, including study design, patient recruitment, and regulatory support. The company focuses on accelerating the development of new therapies by providing integrated solutions across the clinical trial process. Syneos Health is known for its innovative approach to clinical trials, including decentralized trial models and advanced data analytics.
Charles River Laboratories
Charles River Laboratories offers comprehensive preclinical and clinical trial services to the pharmaceutical, biotechnology, and medical device industries. With a strong focus on research and development, the company provides drug discovery, testing, and regulatory services. Charles River’s extensive expertise in clinical trials, particularly in oncology and immunology, makes it a leading player in the North American clinical trials market.
Other key players include Thermo Fisher Scientific Inc., Parexel International, Medpace, Inc., and Frontage Labs.
FAQs
- What is the North American clinical trials market size? The North American clinical trials market was valued at USD 35.31 billion in 2024 and is expected to reach USD 60.16 billion by 2034, growing at a CAGR of 6.0%.
- What are decentralized clinical trials? Decentralized clinical trials (DCTs) are trials that use digital technologies to conduct research remotely, allowing patients to participate without frequent visits to clinical trial sites.
- Which therapeutic areas dominate the clinical trials market? Oncology and neurology are the leading therapeutic areas in North American clinical trials due to the high prevalence of cancer and neurological disorders.
- What challenges are faced by the clinical trials market? Key challenges in the clinical trials market include patient recruitment difficulties, regulatory hurdles, high trial costs, and the need for efficient data management.