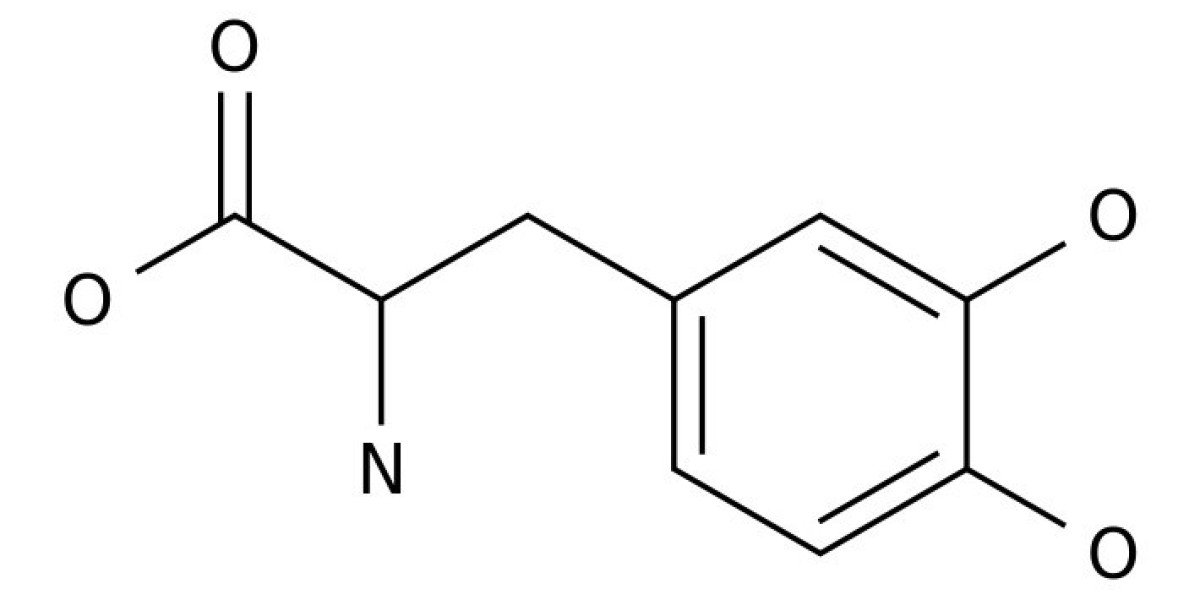
L-3,4-Dihydroxyphenylalanin (DOPA or levodopa) is currently the most used drug to treat symptoms of Parkinson’s disease (PD). After crossing the blood–brain barrier, it is enzymatically converted to dopamine by neuronal cells and restores depleted endogenous neurotransmitter levels. DOPA is prone to auto-oxidation and reactive intermediates of its degradation including reactive oxygen species (ROS) have been implicated in cellular damage. In this study, we investigated how oxygen tension effects DOPA function stability. We applied oxygen tensions comparable to those in the mammalian brain and demonstrated that 2% oxygen almost completely stopped its auto-oxidation. DOPA even exerted a ROS scavenging function. Further mechanistic analysis indicated that DOPA reprogrammed mitochondrial metabolism and reduced oxidative phosphorylation, depolarized the mitochondrial membrane, induced reductive glutamine metabolism, and depleted the NADH pool. These results shed new light on the cellular effects of DOPA function and its neuro-toxicity under physiological oxygen levels that are very distinct to normoxic in vitro conditions.
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease (AD) and affects ~1% of the worldwide population with an age over 65 with raising trend. It is characterized by a substantial loss of dopaminergic neurons within the substantia nigra in the mid brain and results in lower levels of the neurotransmitter dopamine (DA) which is responsible for the classic symptoms like tremor, bradykinesia, rigidity and postural instability. PD patients were firstly treated with levodopa (DOPA) in 1961. In contrast to DA it is able to cross the blood–brain barrier (BBB) due to its amino acid-like structure and therefore bypasses the rate-limiting step of DA synthesis, the DOPA formation via tyrosine hydroxylase (TH). It is converted into DA via aromatic L-amino acid decarboxylase (AADC) and restores DA availability in the brain.
Unfortunately, this kind of treatment only mitigates the symptoms, the process of neurodegeneration is not hindered. The cause of this pathogenesis is currently under intensive investigation, but not yet fully understood. Two of the most discussed hypotheses are related to reactive oxygen species (ROS) and mitochondrial dysfunction. But currently, there is no medication slowing the process of neurodegeneration, so treatment with DOPA function still remains the gold standard and is combined with different co-treatments to optimize the pharmacokinetics of this drug. A critical point in this context is the limited stability of DOPA: Besides enzymatic degradation, there are also spontaneous reactions by which DOPA as well as DA are bound to melanin, an auto-oxidation process during which ROS is formed .