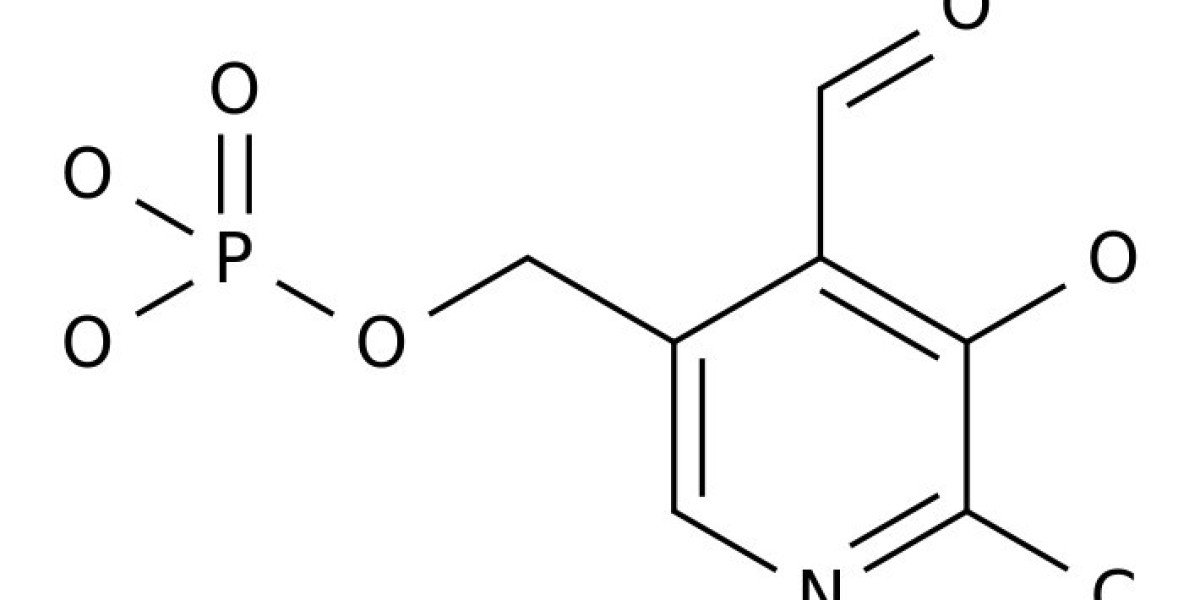
Pyridoxal phosphate (PLP) functions as a coenzyme in many enzymatic processes, including decarboxylation, deamination, transamination, racemization, and others. Enzymes, requiring PLP, are commonly termed PLP-dependent enzymes, and they are widely involved in crucial cellular metabolic pathways in most of (if not all) living organisms. The chemical mechanisms for PLP-mediated reactions have been well elaborated and accepted with an emphasis on the pure chemical steps, but how the chemical steps are processed by enzymes, especially by functions of active site residues, are not fully elucidated. Furthermore, the specific mechanism of an enzyme in relation to the one for a similar class of enzymes seems scarcely described or discussed. This discussion aims to link the specific mechanism described for the individual enzyme to the same types of enzymes from different species with aminotransferases, decarboxylases, racemase, aldolase, cystathionine β-synthase, aromatic phenylacetaldehyde synthase, et al. as models. The structural factors that contribute to the reaction mechanisms, particularly active site residues critical for dictating the reaction specificity, are summarized in this review.
Introduction
Pyridoxal phosphate (PLP) is one of the active forms of vitamin B6, which is produced by pyridoxal kinase-mediated reactions. PLP-dependent enzymes catalyze a wide variety of reaction types and usually have a conserved lysine residue in the active site for PLP binding. The ε-amino group of the lysine residue and the aldehyde group of PLP forms a Schiff-base structure. Because this Schiff-base structure is linked through a protein-associated lysine residue, it is commonly referred as internal aldimine. After substrate (amino acid or amine) binding, the internal aldimine breaks up and a new Schiff base structure is formed between the amino group of substrate and aldehyde group of PLP via a gem-diamine intermediate. This newly formed Schiff base is generally termed external aldimine to distinguish it from Schiff-base structure linked with the lysine residue in proteins.
The importance of the phosphate group in PLP was also studied recently through comparative analysis using pyridoxal and pyridoxal phosphate as the cofactor in serine palmitoyltransferase. Although pyridoxal could associate with a conserved active site lysine residue and transaldimination reactions proceeded, a replacement of PLP with pyridoxal lowered more than 10-fold of its enzyme activity. The phosphate group of PLP was proposed to interact with the substrate L-serine hydroxyl group and contributed to the critical intermediate formation and stereospecific orientation of formed quinonoid or carbanionic intermediates. The phosphate group was also suggested functioning as an acid/base catalyst to promote proton transfer to aid in external aldimine formation and accelerating gem-diol intermediate formation in kynureninase-mediated hydrolytic cleavage reaction.
 AdBlock Detectado
AdBlock Detectado